|
H25: Colorants for Four Colour-Printing
|
©
James H Nobbs
[Colour4Free]
|
|
|
|
By far the largest volumes of
ink are used in three and four colour sets. Because large
amounts are used annually, the cost of the inks and economy of use is a
major consideration. A standard colour, high tinctorial
strength and economy in use are vital characteristics for colorants
that are suitable for process inks.
Major
requirements for four-colour printing
Colour
There are various standards,
such as the Kodak scale and the European norm, which aim to standardise
the colours that can be obtained with fourcolour sets.
Transparency
The order in which the
colours are printed determines the transparency requirement of the
inks. The transparency of the first colour down is less
important, whereas subsequent colours have to possess high
transparency. For offset inks in Europe, yellow is often
printed last and therefore needs to be transparent.
Dispersibility
Transparency may be optimised
through effective dispersion.
The pigments need to have good dispersibility, and to remain stable during the dispersion
process.
Solvent
fastness
Modern mills, for example the
bead mill, generate heat energy and this can cause pigments with poor
solvent fastness to dissolve. On cooling, these types of
pigment will recrystallise, resulting in the loss of colour strength
and transparency. Therefore pigments used in process colours
may be required to have at least moderately good solvent fastness in
the mineral oils or the solvents used in the formulation of heat set
varnishes.
Light
fastness
Light fastness is not
considered to be vital for most publications, but if it is required
pigments such as CI Pigment Yellow 74 can be use for the yellow and CI
Pigment Red 184 for the magenta may be used.
Heat
stability
Heat fastness is not very
important since even heat set inks rarely reach temperatures that
affect the pigments used.
Process
cyan
|
The
cyan standard is always based on CI Pigment Blue 15:3, whose molecular
structure is shown in Figure 1.
Phthalocyanine
pigment was discovered in 1928 by Scottish Dyes (later to become ICI
and now Avecia). Chemists noticed a blue impurity when
heating phthalimide in a ceramic lined vessel that was damaged,
exposing some iron. The impurity proved almost impossible to
destroy. Commercial grades were introduced about ten years
later, complexed around copper rather than iron. Figure 2 shows the
size of particles of the pigment.
|
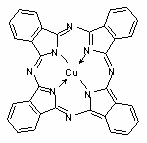
Figure 1: C.I. Pigment Blue 15
|
There
have been some concerns about the copper in CI Pigment Blue 15:3, but
this is so strongly bound within the molecule that it is not
bio-available and therefore is not considered to be an environmental
hazard.
The pigment exists in at
least five crystal forms (α
β γ δ ε), i.e. the
pigment is polymorphic, but only three forms are commercially produced (α β ε).
Pigment 15:3 is the β-phthalocyanine form and a greenish blue shade.
The
pigment has excellent light fastness, as shown in Figure 3
|
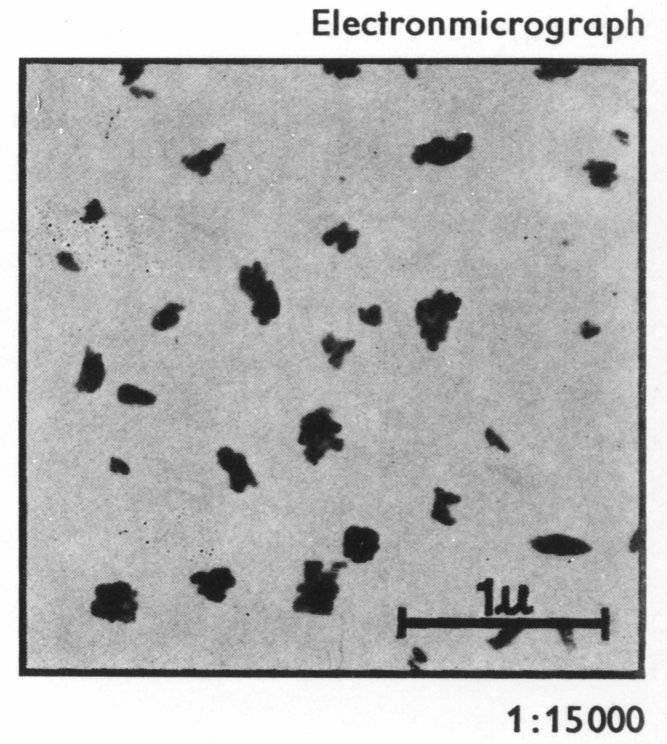
Figure
2: Blue 15: 3 particles
|
Figure
3: Blue 15:3 light fastness panels
|
The
reflectance spectra of offset prints are shown in Figure 4
|
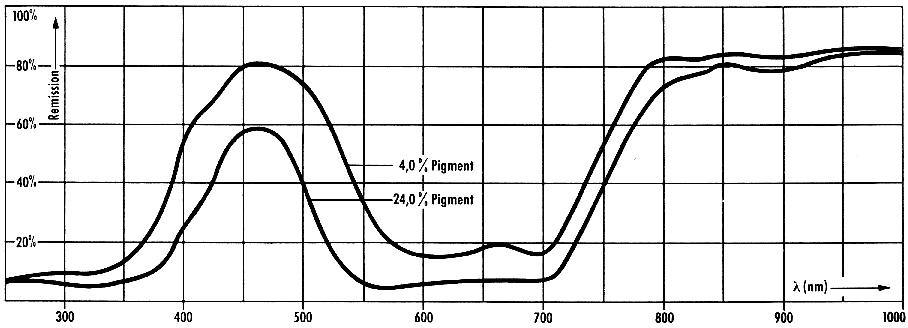
Figure 4: Reflectance spectra of
offset prints of inks containing Pigment Blue 15:3
|
Process yellow
|
The
yellow standard is usually obtained with CI Pigment Yellow 13 (in
Europe), whose molecular structure is shown in Figure 5.
This
pigment is a type of diarylide yellow. They were formerly
known as “benzidine yellows” as they are manufactured from 3, 3’
dichlorobenzidine (DCB).
|
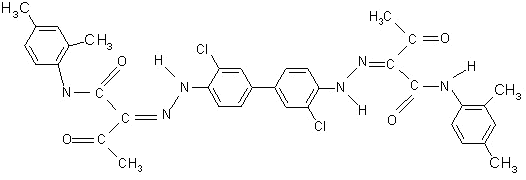
Figure 5: CI Pigment Yellow 13
|
However,
this name should be avoided as it confuses them with benzidine dyes,
over which there are legitimate safety concerns.
In Europe, CI Pigment Yellow
13 is used in most printing inks, in the United States, CI Pigment
Yellow 12 and Yellow 14 predominate. Yellow 13 has a slightly
redder shade, is somewhat stronger and has better fastness properties
than the other two types of pigment.
A
conventional grade of CI Pigment Yellow 13 is not noted for
transparency, special grades are required, based on mixed couplings and
the pigments are often surface treated to ensure the particle size in
the dispersion is minimised. Figure 6 shows the size of
particles of the pigment. The pigment has a high tinctorial
strength, good heat stability and good solvent fastness.
However, the light fastness is poor to moderate as shown in Figure 7.
|
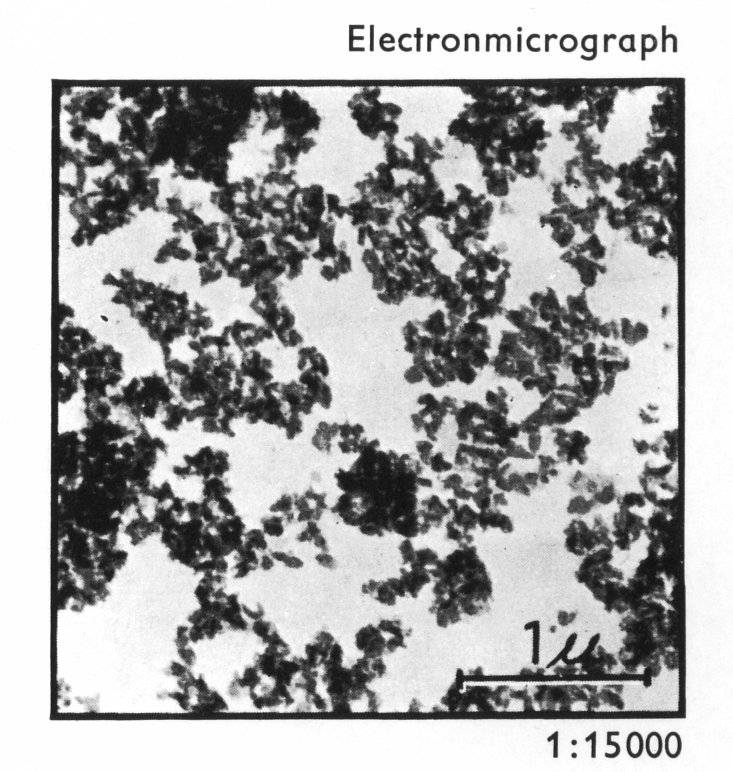
Figure 6: Yellow 13
particles
|
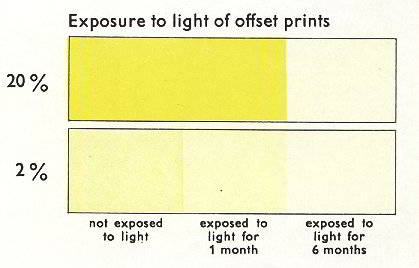
Figure 7: Yellow 13 light
fastness panels
|
The
reflectance spectra of offset prints are shown in Figure 8.
|
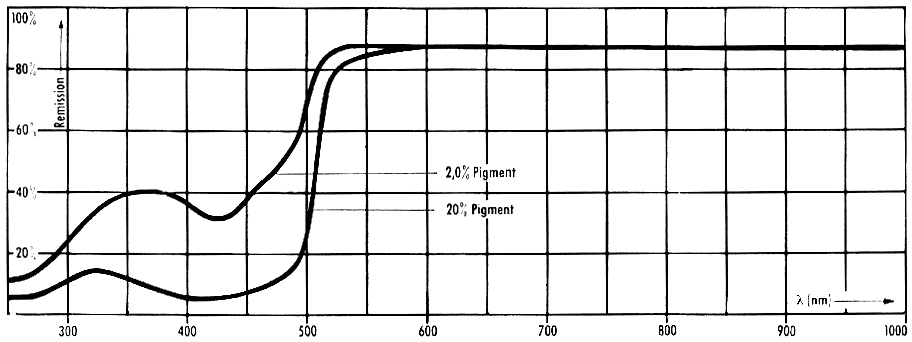
Figure 8: Reflectance
spectra of offset prints of inks containing Pigment Yellow 13
|
Process
magenta
|
The
magenta standard is usually obtained with CI Pigment Red 57:1, whose
molecular structure is shown in Figure 9.
CI
Pigment Red 57:1 (also known as Lithol&®
Rubine, Rubine Toner or Calcium 4B toner) is a toner type of pigment
and this type is one of the easiest to manufacture and one of the most
economic.
|
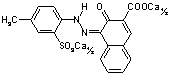
Figure 9: CI Pigment Red 57:1
|
Consequently, they are
particularly useful for printing inks and Red 57:1 is the natural
choice for the magenta standard. Figure 10 shows the size of
particles of the pigment.
The light fastness of toner
type pigments and the shade of toners are influenced by the metal
cation. Calcium (Ca) is the metal cation present in Red
57:1. The light fastness of Red 57:1 is moderate, as
illustrated in Figure 11. The pigment has good heat stability
and moderate solvent fastness.
A
problem with most types of toner pigments is that they are not fast to
alkali. This is also true for Red 57:1, which has relatively
poor fastness to soap and detergents for example.
|
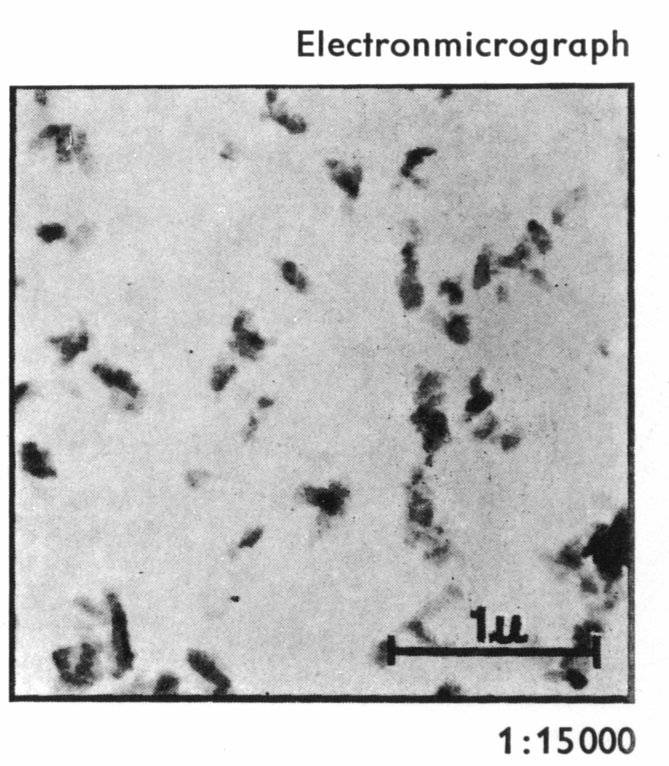
Figure 10: Red 57:1 particles
|
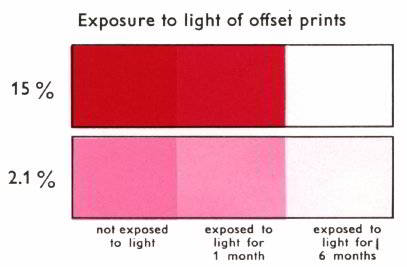
Figure 11: Red 57:1 light
fastness panels
|
The
reflectance spectra of offset prints of inks containing Red 57:1 are
shown in Figure 12.
|
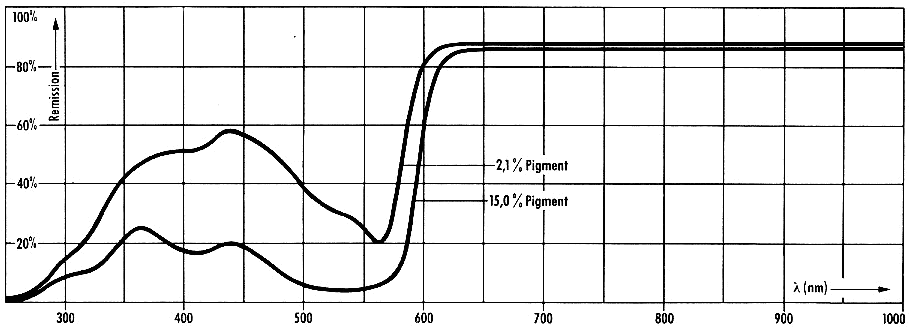
Figure 12: Reflectance
spectra of offset prints of inks containing Pigment Red 57:1
|
Process
black
The black standard is based
on carbon black. Adding a strong blue, such as Alkali Blue or
Iron Blue to the carbon black can improve the jetness of carbon black.
Carbon
black pigments are obtained by burning a suitable carbon rich liquid or
gas with a limited but controlled supply of air. The products
of the incomplete combustions are cooled and collected.
|
Typical
sizes of carbon black pigments range from 0.03 μm
to 0.10 μm depending on the conditions of
production. Figure 13 shows the characteristic spherical
shape of carbon black pigments.
|
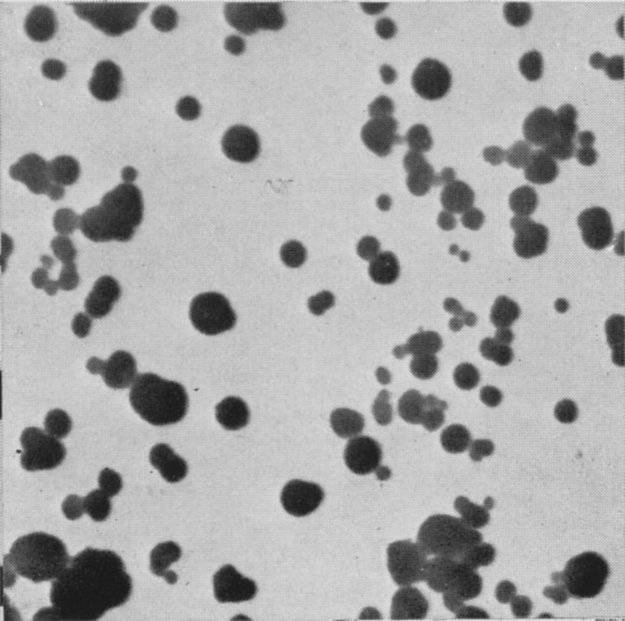
Figure 13: Particles
of a Furnace black. Mag. 16,000
|
Health
and safety
In the UK there is no
legislation dealing with the physiological properties of pigments used
for food packaging, except a duty of care demanding no harm to the
consumer, nor affect in any way the quality of the food being
packaged. However, it is generally understood that coloured
inks should not come into direct contact with food.
CI pigment Red 53:1 (often
used in a secondary colour between magenta and orange) is usually
avoided for children’s publications as it is a type of toner
pigment using barium to produce the salt from the acid dye.
The insolubility and
excellent migration fastness of most organic pigments largely
eliminates human health hazards. However, care may need to be
taken when handling pigments due to the potential presence of
impurities (possibly heavy metals or residual amines). Good
manufacturing procedures and appropriate sample clean up methods help
to ensure that the levels of impurities are minimised. When
pigments are incorporated into formulations it is invariably components
other than pigments that are likely to pose the greatest ecological and
toxicological risks.
|
|
|
|
H25: Colorants for Four Colour-Printing
|
©
James H Nobbs
[Colour4Free]
|












